Should there be a single drug that can miraculously extend human life span given the diverse effects aging has across organs and biological systems?
The answer is yes but if you ask me, way too few people are excited about it.
Let us recap: aging effects everyone but some people live longer than others. When looking at centenarians, the question arises as to the reason for how they die in the end. The answer is that they die of the same stuff that everybody else dies of (cardio-vascular disease, cancer and a diminished immune system unable to fight infections like pneumonia) but centenarians only get those diseases twenty years later.
If we could just push out these diseases for twenty years, we would solve a lot of the problems in our health care system – but how likely is it that we would find a single intervention that does it?
Actually, one such intervention has been with us for centuries and – very topical given that today is Ash Wednesday, the beginning of Lent – is fasting (or caloric restriction).

Religion (especially when it includes adherence to ritual) has gone out of favor and instead, we have replaced true fasting (i.e., not eating) with very modest, metaphorical abstinence during Lent such as this year’s fasting slogan in the German Lutheran Church of „climate fasting“ (supposedly doing good stuff for the climate which can include vacation fasting).
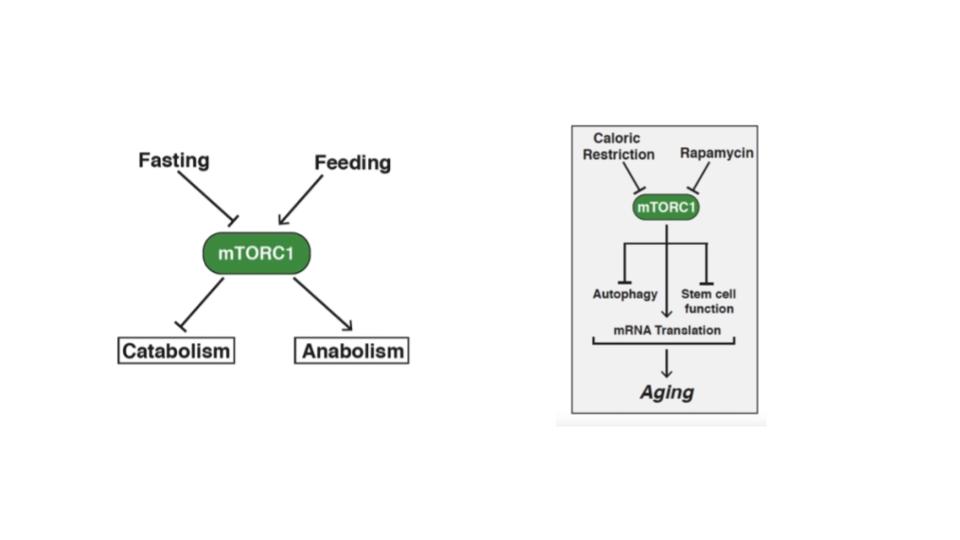
Can a pill provide the same benefit without actually having to do a real fast? Actually, it can and you will not be surprised that the best candidate for such a drug is rapamycin.
Here is the data:
- It dramatically extends longevity in flies, worms and mice (which are very close to us biochemically but live much shorter lives so that you can study longevity in a controlled manner much more easily) and there are some great studies in dogs on its way
- Especially, the mouse data is very convincing given that the data came from genetically heterogeneous mice (i.e., not some breeding artifact) at various laboratories coming to the same conclusion in multiple sites and for multiple study protocols
- It is very likely to have the same life-extending effect in humans and we already know that rapamycin (and its analogues) ameliorate many of the symptoms of aging such as improving immune-system function in humans
- Best of all it is safe, approved for human use (if not for the specific use as an anti-aging drug) by the FDA and EMA and available as a generic (i.e., potentially cheap) since last year
So why do only fitness/life-hack nerds and a few aging researchers talk about rapamycin as a solution to slowing the aging process? Here is a list from Robin Hogarth, a decision scientist, on why people resist „simple“ solutions to complex problems
- People generally believe that complex systems/problems require complex solutions
- New ideas are hard to accept
- It can be difficult to know if simple solutions work
While I believe that the first two are real obstacles (and the additional complicating fact that there is no money in it for the pharmaceutical industry under the current model), I am optimistic regarding the third point.
While it is exceedingly difficult (and expensive) to do randomized-controlled trials with the measured endpoint of lifespan given human longevity, we might not even need it in the current environment.
For me, a relevant case study is the example of the high-fat/low-carbohydrate diet for weight control and reversal of insulin-resistance. While its proponents were clearly seen as cranks just a few years ago, the individual data from people trying it for themselves is so convincing that there is no going back. As a kid growing up in the 80s on a diet of toast and orange juice for breakfast, lots of pasta, very little salt and definitely no eggs and cream I had problems maintaining my weight as an adult despite being an avid runner. Since changing the way I eat about 10 years ago, I have not spent a minute worrying about maintaining my weight and simply eat to satiety twice a day. Do I need a randomized-clinical trial as validation?
After we have all given rapamycin a try to see if the effects are indeed as great as claimed, the pharmaceutical industry might well get back to us due to popular demand as they have recently done in the pharmacological treatment of depression with ketamine. Ketamine, a general anesthetic and popular recreational street drug, was long known for its anti-depressant properties and prescribed on an off-label basis (i.e., not for the indication for which it was originally approved) but is now hailed as the greatest breakthrough in anti-depression therapy in the last 50 years after the regulatory approval of patent-protected nasal spray of a simple enantiomer of ketamine specifically for the treatment of depression – so that there is real money it it again.